Thermo Fisher Crispr Design Tool
What makes the Alt-R CRISPR-Cas9 System so great?
- Length optimization of the crRNAs for increased activity
- Chemical modifications to help increase nuclease resistance and reduce innate immune responses
- Optimized native Cas9 and HiFi Cas9 variant has shown increased specificity in our research
- Two-part guide RNA (crRNA:tracrRNA) provide high activity at an affordable price
- Unique supporting reagents (electroporation enhancer, labeled tracrRNA, controls) provide successful editing
- Complete collection of user-guides and customers-submitted research protocols
Alt-R CRISPR-Cas9 System
Simple delivery of ribonucleoprotein complexes (crRNA:tracrRNA:Cas9 or sgRNA:Cas9).
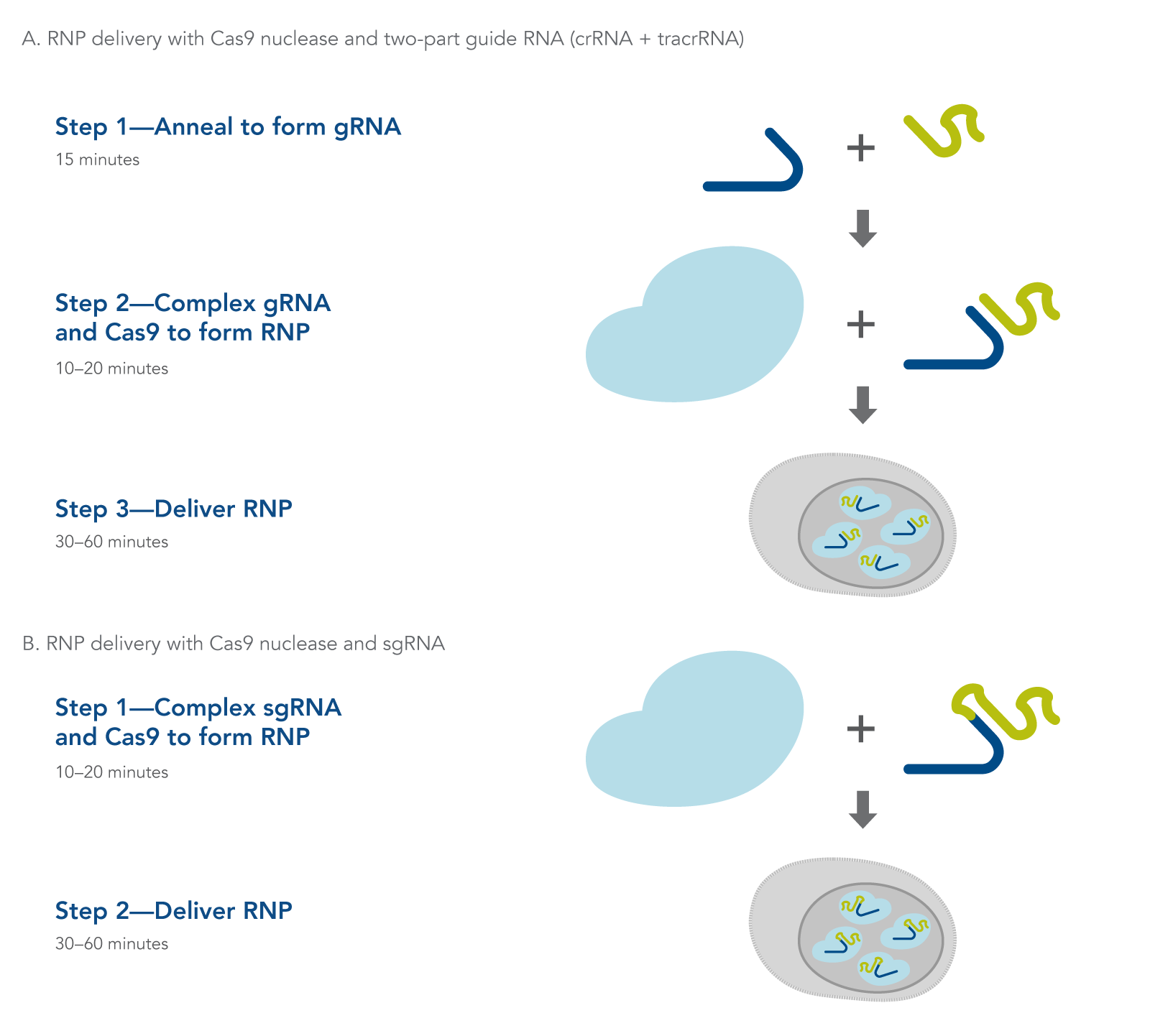
Figure 1. Overview of Alt-R CRISPR-Cas9 System experiments for ribonucleoprotein delivery by lipid-mediated transfection, electroporation, or microinjection using (A) 2-part guide RNAs (crRNA:tracrRNA duplex) or (B) sgRNAs.
CRISPR-Cas9 genome editing methods use a Cas9 endonuclease to generate double-stranded breaks in DNA. Cas9 endonuclease requires a CRISPR RNA (crRNA) to specify the DNA target sequence, and the crRNA must be combined with the transactivating crRNA (tracrRNA) to activate the endonuclease and create a functional editing ribonucleoprotein complex (Figure 1A). In an alternative approach, the crRNA and tracrRNA can be delivered as a single RNA oligonucleotide (Figure 1B). After cleavage, DNA is then repaired by non-homologous end-joining (NHEJ) or homology-directed recombination (HDR), resulting in a modified sequence. Alt-R CRISPR-Cas9 reagents and kits provide essential, optimized tools needed to use this pathway for genome editing research.
| Alt-R CRISPR-Cas9 System | |
|---|---|
| Ribonucleoprotein components | |
| Option 1: Alt-R CRISPR-Cas9 crRNA:tracrRNA | Alt-R CRISPR-Cas9 crRNA
|
| Alt-R CRISPR-Cas9 tracrRNA
| |
| Option 2: Alt-R CRISPR-Cas9 sgRNA |
|
| Alt-R S.p. Cas9 Nuclease/Nickase |
|
| Additional reagents and kits | |
|---|---|
| Alt-R CRISPR-Cas9 Control Kits |
|
| Alt-R CRISPR-Cas9 Electroporation Enhancer | For primary and difficult-to-transfect cells |
| Alt-R HDR Enhancer V2 | For improved rates of homology-directed repair |
| Alt-R Genome Editing Detection Kit | For mutation detection and estimating editing efficiency |
Ribonucleoprotein components
The Alt-R CRISPR-Cas9 System offers two options for generating synthetic guide RNAs. The two-part system pairs an optimized, shortened universal tracrRNA oligonucleotide (67 nt) with an optimized, shortened, target-specific crRNA oligonucleotide (36 nt) for improved targeting of Cas9 to dsDNA targets (Figure 2). The single guide RNA (sgRNA) option combines the crRNA and tracrRNA segments into one long RNA molecule, reducing the number of components and simplifying the CRISPR workflow.
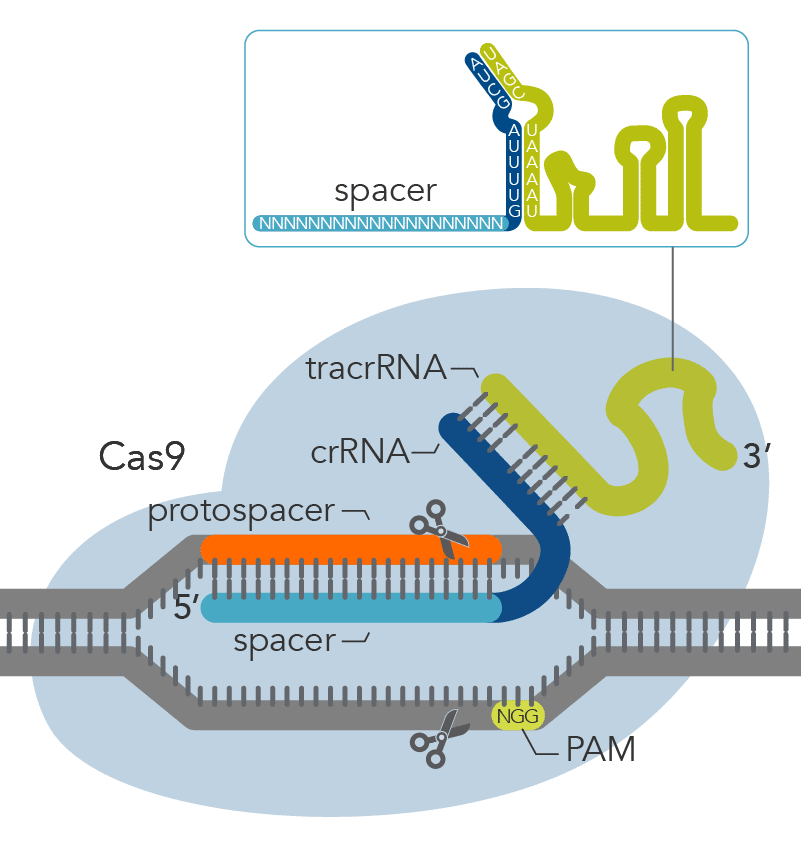
Figure 2. Components of the Alt-R CRISPR-Cas9 System for directing Cas9 endonuclease to genomic targets. For the 2-part guide RNA format, the crRNA:tracrRNA complex uses optimized Alt-R crRNA and tracrRNA sequences that hybridize and then form a complex with Cas9 endonuclease to guide targeted cleavage of genomic DNA. Alternatively, the sgRNA (not shown), which contains relevant crRNA and tracrRNA sequences, forms a complex with Cas9 endonuclease to guide targeted cleavage of genomic DNA. The cleavage site is specified by the spacer element of the crRNA (light blue bar). The crRNA spacer element recognizes 19 or 20 nt on the strand opposite from the NGG PAM site. The PAM site must be present immediately downstream of the protospacer element for cleavage to occur. Research by IDT scientists has shown that the Alt-R CRISPR-Cas9 System provides the highest percentage of on-target genome editing when compared to competing designs of native S. pyogenes crRNA:tracrRNA.
While delivering Cas9 nuclease as part of an RNP is the preferred method, the Alt-R CRISPR-Cas9 System is also compatible with S. pyogenes Cas9 from any source, including cells that stably express S. pyogenes Cas9 endonuclease, or when Cas9 is introduced as a DNA or mRNA construct.
Option 1 (2-part guide RNA)
- Alt-R CRISPR-Cas9 crRNA
All Alt-R CRISPR-Cas9 crRNAs are 35–36 nt RNA oligos containing the 19 or 20 nt target-specific protospacer region, along with the 16 nt tracrRNA fusion domain. We recommend 20 nt protospacers for most applications. crRNAs should be duplexed with Alt-R CRISPR-Cas9 tracrRNA before RNP complex formation.
Alt-R CRISPR-Cas9 crRNAs are synthesized with proprietary chemical modifications, which protect the crRNA from degradation by cellular RNases and further improve on-target editing performance. When using 2-part gRNAs under highly challenging conditions (e.g., high nuclease environments or with Cas9 mRNA), use Alt-R CRISPR-Cas9 crRNA XT, which have additional chemical modifications for the highest level of stability and function.
We guarantee* our predesigned guide RNAs targeting human, mouse, rat, zebrafish, or nematode genes. For other species, we recommend using our proprietary algorithms to design custom guide RNAs. If you have protospacer designs of your own or from publications, use our design checker tool to assess their on- and off-targeting potential before ordering guide RNAs that are synthesized using our Alt-R guide RNA modifications.
* See Ordering section for details.
- Alt-R CRISPR-Cas9 tracrRNA
The 67 nt Alt-R tracrRNA is much shorter than the classical 89 bases of the natural S. pyogenes tracrRNA. We find that shortening the tracrRNA increases on-target performance. Alt-R CRISPR tracrRNA also contains proprietary chemical modifications that confer increased nuclease resistance.
Alt-R CRISPR-Cas9 tracrRNA labeled with ATTO™ 550 (ATTO-TEC) provide the same function as their unlabeled counterparts. However, the fluorescent dye allows you to monitor transfection or electroporation efficiency during preliminary experiments to optimize transfection conditions in your cell types (Figure 3).

Figure 3. Detection of fluorescently labeled tracrRNA by fluorescence microscopy. HEK-293 cells stably expressing Cas9 nuclease were reverse transfected (RNAiMAX reagent, Thermo Fisher Scientific) with Alt-R CRISPR-Cas9 crRNA (unlabeled) complexed with Alt-R CRISPR-Cas9 tracrRNA – ATTO 550 (final concentration of 10 nM). Images were taken 48 hr after transfection. Magnification: 10X.
Labeled tracrRNAs can also help concentrate transfected cells via FACS (fluorescence-activated cell sorting) analysis, which can simplify your screening process for cells with CRISPR events. (For more information and tips on using Alt-R CRISPR-Cas9 tracrRNA – ATTO 550, see the application note.)
Alt-R CRISPR tracrRNA orders include Nuclease-Free Duplex Buffer for forming the complex between crRNA and tracrRNA oligos. Alt-R tracrRNA can be ordered in larger scale and paired with all of your target specific crRNAs, allowing for an easy and a cost-effective means of studying many CRISPR sites.
Option 2 (single guide RNA)
Alt-R CRISPR-Cas sgRNA
Alt-R CRISPR-Cas9 sgRNAs are long RNA oligonucleotides (99–100 bases) containing the target-specific crRNA region and the Cas9-interacting tracrRNA region within a single molecule (i.e., 19–20 base protospacer region and 80-base universal sgRNA region). Like other Alt-R RNAs, it contains chemical modifications to stabilize the RNA, increasing resistance to nuclease activity. For challenging conditions (e.g., high nuclease environments or with Cas9 mRNA), sgRNAs may provide increased potency.
Cas9 endonucleases
- Alt-R S.p Cas9 nuclease
The Alt-R S.p. Cas9 Nuclease V3 enzyme is a high purity, recombinant S. pyogenes Cas9. The enzymes include nuclear localization sequences (NLSs) and C-terminal 6-His tags. The S. pyogenes Cas9 enzyme must be combined with a gRNA to produce a functional, target-specific editing complex. For the best editing, combine the Alt-R S.p. Cas9 Nuclease V3 enzyme with the optimized Alt-R CRISPR gRNA in equimolar amounts.
- Alt-R S.p. HiFi Cas9 nuclease
The Alt-R S.p. HiFi Cas9 Nuclease V3 offers improved specificity over wild-type Cas9, greatly reducing the risk of off-target cutting events. This Cas9 variant also preserves the high level of editing efficiency expected from a Cas9 nuclease, maintaining 90–100% on-target editing activity at most sites. For applications that are sensitive to off-target events, combining the Alt-R S.p. HiFi Cas9 Nuclease V3 with optimized Alt-R CRISPR-Cas9 gRNA (crRNA:tracrRNA) is highly recommended.
- Alt-R S.p. Cas9-GFP (or RFP) nuclease
The Alt-R S.p. Cas9-GFP V3 and S.p. Cas9-RFP V3 nucleases are high purity, recombinant S. pyogenes Cas9 enzymes that are expressed as fusion proteins with nuclear localization sequences (NLSs) and C-terminal 6-His tags. These enzymes have on-target functionality comparable to wild-type S.p. Cas9 and are designed for applications that require post-transfection visualization of the protein or enrichment of edited cells using fluorescence-activated cell sorting (FACS). These enzymes should be combined with Alt-R CRISPR gRNA in equimolar amounts.
- Alt-R S.p. Cas9 nickases
Cas9 nickases allow specific cutting of only one strand at the DNA target site. Cuts to both strands of DNA are accomplished by using either Alt-R S.p. Cas9 D10A Nickase V3 or Alt-R S.p. Cas9 H840A Nickase V3, with 2 gRNAs that target two neighboring Cas9 sites, one on either strand of the target region. This functionally increases the length of the recognition sequence from 20 to 40 bases. For more information about using Cas9 nickases, see the application note.
- Alt-R S.p. dCas9 protein
Alt-R S.p. dCas9 Protein V3 has mutations that result in the loss of nuclease activity. This protein can form RNP complexes with Alt-R gRNAs and bind to the target region specified by the gRNA without cutting the DNA.
Like the other Alt-R enzymes, Alt-R S.p. dCas9 Protein V3 is provided as 10 mg/mL in 50% glycerol, and it can be diluted in PBS or Opti-MEM® media (Thermo Fisher) before use.
- Alt-R S.p. Cas9 Expression Plasmid
In some cases, transfection of RNP or the creation of stably transfected cells is not possible. In those applications, Alt‑R S.p. Cas9 Expression Plasmid is designed to provide expression of Cas9 endonuclease under CMV promoter control. Note that the plasmid contains no eukaryotic selectable marker, making expression of S.p. Cas9 transient. The Alt-R CRISPR-Cas9 System Plasmid User Guide provides instructions for using this plasmid.
Cas9 comparison chart
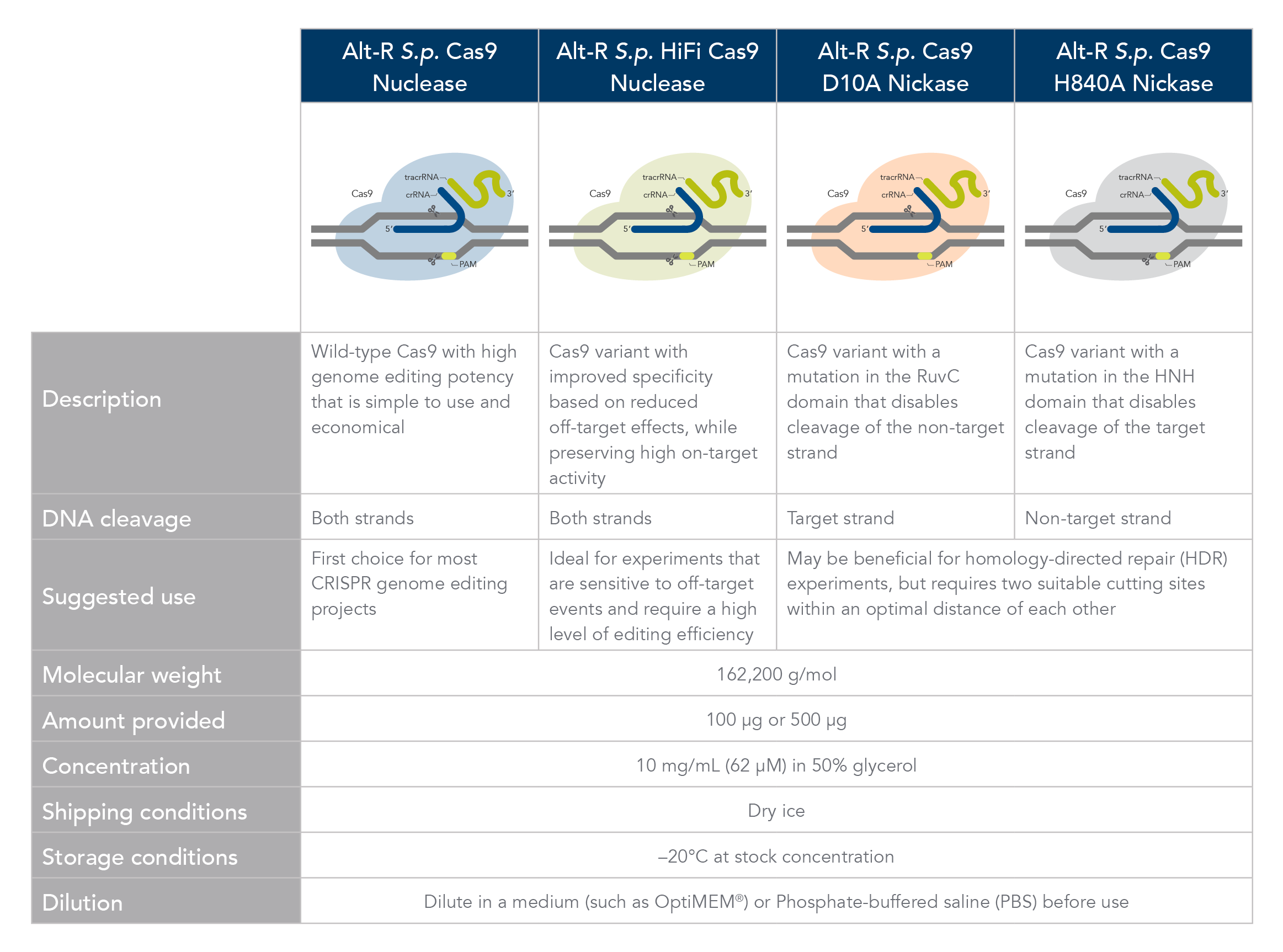
Comparison of Alt-R Cas9 nucleases and nickases.
Homology-directed repair reagents
Alt-R HDR Donor Oligos
Alt-R HDR Donor Oligos have been developed specifically for insertion into DNA by HDR. These HDR templates have enhanced stability and higher rates of incorporation than standard oligonucleotides.
- Exceptional flexibility: multiple species, as well as single or multiple (batch) design modes
- Compatible with Cas9 nucleases (double-stranded cleavage) or nickases (single-stranded cleavage)
- Accommodates incorporation of desired sequence (such as sequence tags, stop codons, GFP, enhancers, or promoters) into target site
Alt-R HDR Enhancer V2
Alt-R HDR Enhancer is a small molecule compound that increases homology-directed repair. Alt-R HDR Enhancer V2 is a new and improved small molecule compound that exhibits its activity in multiple cell lines, including both adherent and suspension cell lines. Its activity is independent of the enzyme employed; for example, it can be used either with Alt-R S.p. Cas9 nucleases or A.s. Cas12a (Cpf1) nucleases. This versatile reagent is also compatible with electroporation and lipofection methods.
Additional reagents and kits
Alt-R CRISPR-Cas9 Control crRNAs and PCR Assays
Optional controls for human, mouse, and rat are available for the 2-part Alt-R CRISPR-Cas9 System.
We recommend using the appropriate Alt-R CRISPR-Cas9 Control Kit for studies in human, mouse, or rat cells. The control kits include an Alt-R CRISPR HPRT Positive Control crRNA targeting the HPRT (hypoxanthine phosphoribosyltransferase) gene and a computationally confirmed Alt-R CRISPR-Cas9 Negative Control crRNA. The kit also includes the Alt-R CRISPR-Cas9 tracrRNA for complexing with the crRNA controls, Nuclease-Free Duplex Buffer, and validated PCR primers for amplifying the targeted HPRT region in the selected organism. The inclusion of the PCR assay makes the kits ideal for confirmation of HPRT modification using the Alt-R Genome Editing Detection Kit.
Alt-R control kit components can also be ordered individually.
For information about sgRNA controls, contact applicationsupport@idtdna.com.
Alt-R Cas9 Electroporation Enhancer
If you are studying primary or hard-to-transfect cells, electroporation is often a viable alternative to lipid-based transfection in CRISPR experiments. The Alt-R Cas9 Electroporation Enhancer is a Cas9-specific carrier DNA that is optimized to work with the Amaxa® Nucleofector® device (Lonza) and Neon® System (Thermo Fisher) to increase transfection efficiency and thereby increase genome editing efficiency (Figure 4).
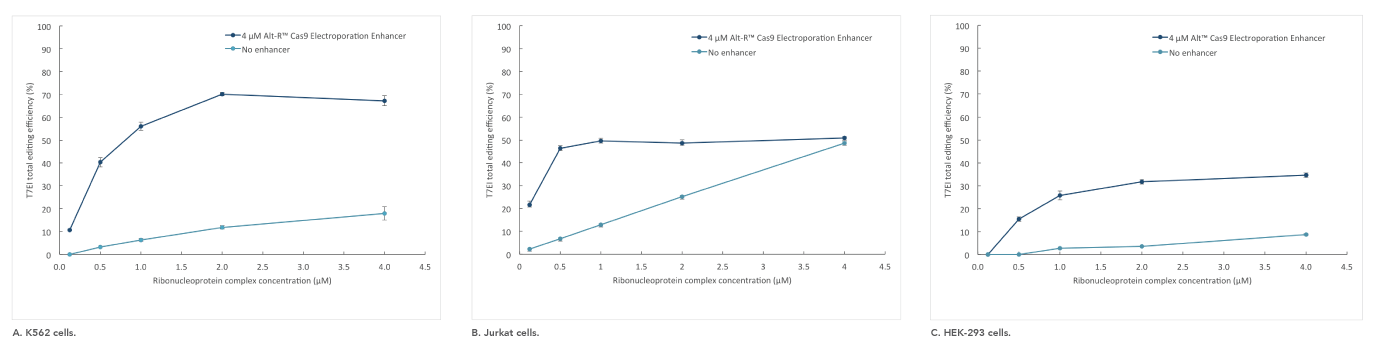
Figure 4. Alt-R Cas9 Electroporation Enhancer improves CRISPR editing efficiency in ribonucleoprotein (RNP) electroporation experiments. K562 (A), Jurkat (B), and HEK-293 (C) cells were transfected (Amaxa System, Lonza) with 0.125–4 µM RNP (Alt-R S.p. Nuclease 3NLS complexed with Alt-R CRISPR-Cas9 crRNA and tracrRNA). Electroporation reactions were performed in the presence (dark blue) or absence (light blue) of 4 µM Alt-R Cas9 Electroporation Enhancer.
Alt-R Genome Editing Detection Kit
Use this kit to detect on-target genome editing and estimate genome editing efficiency in CRISPR experiments. Learn more >>
Thermo Fisher Crispr Design Tool
Source: https://www.idtdna.com/pages/products/crispr-genome-editing/alt-r-crispr-cas9-system
Posted by: fierropornat.blogspot.com

0 Response to "Thermo Fisher Crispr Design Tool"
Post a Comment